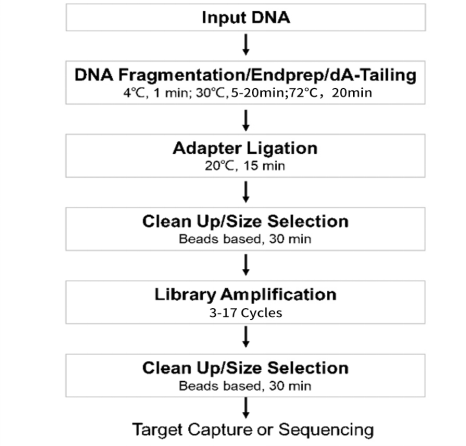
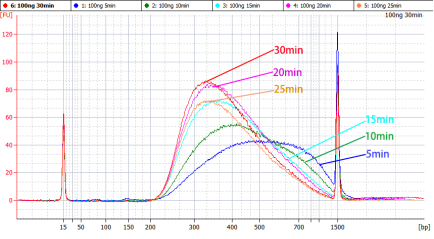
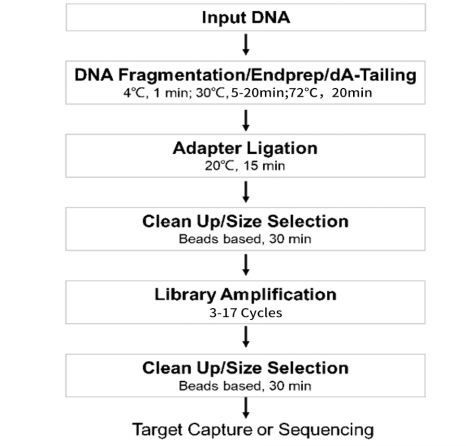
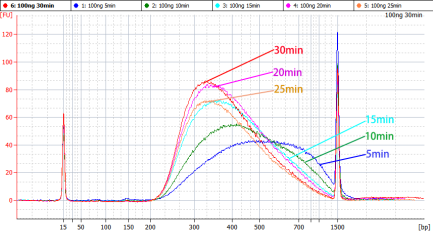
[1] Wang Y, Wang Y, Song D, et al. An RNA-cleaving threose nucleic acid enzyme capable of single point mutation discrimination. Nat Chem. 2022;14(3):350-359. doi:10.1038/s41557-021-00847-3(IF:24.427)
[2] Zhang C, Zhou B, Gu F, et al. Micropeptide PACMP inhibition elicits synthetic lethal effects by decreasing CtIP and poly(ADP-ribosyl)ation. Mol Cell. 2022;82(7):1297-1312.e8. doi:10.1016/j.molcel.2022.01.020(IF:17.970)
[3] Qiao J, Jiang H, Lin Y, et al. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice. Mol Plant. 2021;14(10):1683-1698. doi:10.1016/j.molp.2021.06.023(IF:13.164)
[4] Sun X, Peng X, Cao Y, Zhou Y, Sun Y. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling. Nat Commun. 2020;11(1):2984. Published 2020 Jun 12. doi:10.1038/s41467-020-16799-0(IF:11.614)
[5] Ma D, Zhao Y, Huang L, et al. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials. 2020;237:119830. doi:10.1016/j.biomaterials.2020.119830(IF:10.317)
[6] Wang X, Chen K, Zhou M, et al. GmNAC181 promotes symbiotic nodulation and salt tolerance of nodulation by directly regulating GmNINa expression in soybean [published online ahead of print, 2022 Jun 25]. New Phytol. 2022;10.1111/nph.18343. doi:10.1111/nph.18343(IF:10.152)
[7] Yun J, Sun Z, Jiang Q, et al. The miR156b-GmSPL9d module modulates nodulation by targeting multiple core nodulation genes in soybean. New Phytol. 2022;233(4):1881-1899. doi:10.1111/nph.17899(IF:10.152)
[8] Yan WH, Wu MY, Shah S, et al. Silencing the triacylglycerol lipase (TGL) gene decreases the number of apyrene sperm and inhibits oviposition in Sitotroga cerealella. Cell Mol Life Sci. 2021;79(1):44. Published 2021 Dec 31. doi:10.1007/s00018-021-04048-6(IF:9.261)
[9] Tang M, Bai L, Wan Z, et al. circRNA-DURSA regulates trophoblast apoptosis via miR-760-HIST1H2BE axis in unexplained recurrent spontaneous abortion. Mol Ther Nucleic Acids. 2021;26:1433-1445. Published 2021 Jun 24. doi:10.1016/j.omtn.2021.06.012(IF:8.886)
[10] Zhang J, Zhao Y, Tian Y, et al. Genome-wide screening in the haploid system reveals Slc25a43 as a target gene of oxidative toxicity. Cell Death Dis. 2022;13(3):284. Published 2022 Mar 30. doi:10.1038/s41419-022-04738-4(IF:8.469)
[11] He D, Ma Z, Xue K, Li H. Juxtamembrane 2 mimic peptide competitively inhibits mitochondrial trafficking and activates ROS-mediated apoptosis pathway to exert anti-tumor effects. Cell Death Dis. 2022;13(3):264. Published 2022 Mar 24. doi:10.1038/s41419-022-04639-6(IF:8.469)
[12] An J, Feng L, Ren J, et al. Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating β-adrenergic signaling. Oncoimmunology. 2021;10(1):2004659. Published 2021 Nov 23. doi:10.1080/2162402X.2021.2004659(IF:8.110)
[13] Wu H, Zhan T, Cui S, et al. Endothelial barrier dysfunction induced by anthracene and its nitrated or oxygenated derivatives at environmentally relevant levels. Sci Total Environ. 2022;802:149793. doi:10.1016/j.scitotenv.2021.149793(IF:7.963)
[14] Ou Z, Ma Y, Sun Y, et al. A GPR17-cAMP-Lactate Signaling Axis in Oligodendrocytes Regulates Whole-Body Metabolism. Cell Rep. 2019;26(11):2984-2997.e4. doi:10.1016/j.celrep.2019.02.060(IF:7.815)
[15] Wang Y, You K, You Y, et al. Paeoniflorin prevents aberrant proliferation and differentiation of intestinal stem cells by controlling C1q release from macrophages in chronic colitis [published online ahead of print, 2022 Jun 16]. Pharmacol Res. 2022;182:106309. doi:10.1016/j.phrs.2022.106309(IF:7.658)
[16] Shen M, Guo M, Li Y, et al. m6A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free Radic Biol Med. 2022;182:246-259. doi:10.1016/j.freeradbiomed.2022.02.028(IF:7.376)
[17] Li X, Liu J, Lu L, et al. Sirt7 associates with ELK1 to participate in hyperglycemia memory and diabetic nephropathy via modulation of DAPK3 expression and endothelial inflammation. Transl Res. 2022;247:99-116. doi:10.1016/j.trsl.2022.04.005(IF:7.012)
[18] Duan Y, Jiang N, Chen J, Chen J. Expression, localization and metabolic function of "resurrected" human urate oxidase in human hepatocytes. Int J Biol Macromol. 2021;175:30-39. doi:10.1016/j.ijbiomac.2021.01.163(IF:6.953)
[19] Zhou M, Li B, Liu J, Hong L. Genomic, Immunological, and Clinical Characterization of Pyroptosis in Ovarian Cancer. J Inflamm Res. 2021;14:7341-7358. Published 2021 Dec 24. doi:10.2147/JIR.S344554(IF:6.922)
[20] Yang P, Chen T, Wu K, et al. A homozygous variant in TBPL2 was identified in women with oocyte maturation defects and infertility. Hum Reprod. 2021;36(7):2011-2019. doi:10.1093/humrep/deab094(IF:6.918)
[21] Li Y, Dong Y, Ran Y, et al. Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res Ther. 2021;12(1):358. Published 2021 Jun 21. doi:10.1186/s13287-021-02416-4(IF:6.832)
[22] Peng L, Wang Y, Yang B, Qin Q, Song E, Song Y. Polychlorinated biphenyl quinone regulates MLKL phosphorylation that stimulates exosome biogenesis and secretion via a short negative feedback loop. Environ Pollut. 2021;274:115606. doi:10.1016/j.envpol.2020.115606(IF:6.793)
[23] Lv J, Zheng T, Song Z, et al. Strawberry Proteome Responses to Controlled Hot and Cold Stress Partly Mimic Post-harvest Storage Temperature Effects on Fruit Quality. Front Nutr. 2022;8:812666. Published 2022 Feb 15. doi:10.3389/fnut.2021.812666(IF:6.576)
[24] Liu W, Guan Y, Qiao S, et al. Antiaging Effects of Vicatia thibetica de Boiss Root Extract on Caenorhabditis elegans and Doxorubicin-Induced Premature Aging in Adult Mice. Oxid Med Cell Longev. 2021;2021:9942090. Published 2021 Aug 6. doi:10.1155/2021/9942090(IF:6.543)
[25] Liu S, Wu W, Chen Q, et al. TXNRD1: A Key Regulator Involved in the Ferroptosis of CML Cells Induced by Cysteine Depletion In Vitro. Oxid Med Cell Longev. 2021;2021:7674565. Published 2021 Dec 7. doi:10.1155/2021/7674565(IF:6.543)
[26] Liang X, Wang Y, Liu L, et al. Acute nitrite exposure interferes with intestinal thyroid hormone homeostasis in grass carp (Ctenopharyngodon idellus). Ecotoxicol Environ Saf. 2022;237:113510. doi:10.1016/j.ecoenv.2022.113510(IF:6.291)
[27] Duan N, Zhang Y, Tan S, et al. Therapeutic targeting of STING-TBK1-IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol Dis. 2022;169:105739. doi:10.1016/j.nbd.2022.105739(IF:5.996)
[28] Wu YR, Shi XY, Ma CY, Zhang Y, Xu RX, Li JJ. Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc Diabetol. 2019;18(1):146. Published 2019 Nov 9. doi:10.1186/s12933-019-0954-6(IF:5.948)
[29] Li J, Teng P, Yang F, Ou X, Zhang J, Chen W. Bioinformatics and Screening of a Circular RNA-microRNA-mRNA Regulatory Network Induced by Coxsackievirus Group B5 in Human Rhabdomyosarcoma Cells. Int J Mol Sci. 2022;23(9):4628. Published 2022 Apr 22. doi:10.3390/ijms23094628(IF:5.924)
[30] Li J, Teng P, Yang F, Ou X, Zhang J, Chen W. Bioinformatics and Screening of a Circular RNA-microRNA-mRNA Regulatory Network Induced by Coxsackievirus Group B5 in Human Rhabdomyosarcoma Cells. Int J Mol Sci. 2022;23(9):4628. Published 2022 Apr 22. doi:10.3390/ijms23094628(IF:5.924)
[31] Zhang W, Ho CT, Lu M. Piperine Improves Lipid Dysregulation by Modulating Circadian Genes Bmal1 and Clock in HepG2 Cells. Int J Mol Sci. 2022;23(10):5611. Published 2022 May 17. doi:10.3390/ijms23105611(IF:5.924)
[32] Xiang C, Fan C, Lu Q, et al. Interfering with alternatively activated macrophages by CSF-1R inhibition exerts therapeutic capacity on allergic airway inflammation. Biochem Pharmacol. 2022;198:114952. doi:10.1016/j.bcp.2022.114952(IF:5.858)
[33] Xia S, Wang Z, Chen L, et al. Dihydroartemisinin regulates lipid droplet metabolism in hepatic stellate cells by inhibiting lncRNA-H19-induced AMPK signal. Biochem Pharmacol. 2021;192:114730. doi:10.1016/j.bcp.2021.114730(IF:5.858)
[34] Liu N, Meng F, Tian C. Transcriptional Network in Colletotrichum gloeosporioides Mutants Lacking Msb2 or Msb2 and Sho1. J Fungi (Basel). 2022;8(2):207. Published 2022 Feb 21. doi:10.3390/jof8020207(IF:5.816)
[35] Chen Y, Chen J, Shu A, et al. Combination of the Herbs Radix Rehmanniae and Cornus Officinalis Mitigated Testicular Damage From Diabetes Mellitus by Enhancing Glycolysis via the AGEs/RAGE/HIF-1α Axis. Front Pharmacol. 2021;12:678300. Published 2021 Jun 28. doi:10.3389/fphar.2021.678300(IF:5.811)
[36] Dai Z, Cai L, Chen Y, et al. Brusatol Inhibits Proliferation and Invasion of Glioblastoma by Down-Regulating the Expression of ECM1. Front Pharmacol. 2021;12:775680. Published 2021 Dec 14. doi:10.3389/fphar.2021.775680(IF:5.811)
[37] Li H, Fan C, Lu H, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions. Acta Pharm Sin B. 2020;10(3):447-461. doi:10.1016/j.apsb.2019.08.006(IF:5.808)
[38] Fan X, Li Y, Yi X, et al. Epigenome-wide DNA methylation profiling of portal vein tumor thrombosis (PVTT) tissues in hepatocellular carcinoma patients. Neoplasia. 2020;22(11):630-643. doi:10.1016/j.neo.2020.09.007(IF:5.696)
[39] Rong R, Yang R, Li H, et al. The roles of mitochondrial dynamics and NLRP3 inflammasomes in the pathogenesis of retinal light damage. Ann N Y Acad Sci. 2022;1508(1):78-91. doi:10.1111/nyas.14716(IF:5.691)
[40] Ye S, Yang N, Lu T, et al. Adamts18 modulates the development of the aortic arch and common carotid artery. iScience. 2021;24(6):102672. Published 2021 May 31. doi:10.1016/j.isci.2021.102672(IF:5.458)
[41] Li G, Yang L, Feng L, et al. Syringaresinol Protects against Type 1 Diabetic Cardiomyopathy by Alleviating Inflammation Responses, Cardiac Fibrosis, and Oxidative Stress. Mol Nutr Food Res. 2020;64(18):e2000231. doi:10.1002/mnfr.202000231(IF:5.309)
[42] Hu Y, Liu M, Qin H, et al. Artemether, Artesunate, Arteannuin B, Echinatin, Licochalcone B and Andrographolide Effectively Inhibit SARS-CoV-2 and Related Viruses In Vitro. Front Cell Infect Microbiol. 2021;11:680127. Published 2021 Aug 30. doi:10.3389/fcimb.2021.680127(IF:5.293)
[43] Xu B, Zhang C, Jiang A, et al. Histone methyltransferase Dot1L recruits O-GlcNAc transferase to target chromatin sites to regulate histone O-GlcNAcylation. J Biol Chem. 2022;298(7):102115. doi:10.1016/j.jbc.2022.102115(IF:5.157)
[44] Cai X, Tian F, Teng L, et al. Cultivation of a Lytic Double-Stranded RNA Bacteriophage Infecting Microvirgula aerodenitrificans Reveals a Mutualistic Parasitic Lifestyle. J Virol. 2021;95(17):e0039921. doi:10.1128/JVI.00399-21(IF:5.103)
[45] Qi J, Chen X, Wu Q, et al. Fasting Induces Hepatocellular Carcinoma Cell Apoptosis by Inhibiting SET8 Expression. Oxid Med Cell Longev. 2020;2020:3985089. Published 2020 Mar 19. doi:10.1155/2020/3985089(IF:5.076)
[46] Wang J, Han C, Lu Z, et al. Simulated microgravity suppresses MAPK pathway-mediated innate immune response to bacterial infection and induces gut microbiota dysbiosis. FASEB J. 2020;34(11):14631-14644. doi:10.1096/fj.202001428R(IF:4.966)
[47] Yang L, Wang X, Jiao X, et al. Suppressor of Ty 16 promotes lung cancer malignancy and is negatively regulated by miR-1227-5p. Cancer Sci. 2020;111(11):4075-4087. doi:10.1111/cas.14627(IF:4.966)
[48] Wang ZM, Xia SW, Zhang T, et al. LncRNA-H19 induces hepatic stellate cell activation via upregulating alcohol dehydrogenase III-mediated retinoic acid signals. Int Immunopharmacol. 2020;84:106470. doi:10.1016/j.intimp.2020.106470(IF:4.932)
[49] Zhou M, Li B, Liu C, et al. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(Pt B):108223. doi:10.1016/j.intimp.2021.108223(IF:4.932)
[50] Chen X, Yao T, Cai J, et al. A novel cis-regulatory variant modulating TIE1 expression associated with attention deficit hyperactivity disorder in Han Chinese children. J Affect Disord. 2022;300:179-188. doi:10.1016/j.jad.2021.12.066(IF:4.839)
[51] Chen DD, Fang BZ, Manzoor A, et al. Revealing the salinity adaptation mechanism in halotolerant bacterium Egicoccus halophilus EGI 80432T by physiological analysis and comparative transcriptomics. Appl Microbiol Biotechnol. 2021;105(6):2497-2511. doi:10.1007/s00253-021-11190-5(IF:4.813)
[52] Li X, Yuan S, Sun Z, et al. Gene identification and functional analysis of peptidoglycan recognition protein from the spotted sea bass (Lateolabrax maculatus). Fish Shellfish Immunol. 2020;106:1014-1024. doi:10.1016/j.fsi.2020.08.041(IF:4.581)
[53] Zhao X, Huang W, Guo J, et al. PLAAT1 promotes p53 degradation via autophagy-lysosome pathway in zebrafish. Fish Shellfish Immunol. 2022;125:48-53. doi:10.1016/j.fsi.2022.05.001(IF:4.581)
[54] Wang W, Wang J, Lei L, et al. Characterisation of IL-15 and IL-2Rβ in grass carp: IL-15 upregulates cytokines and transcription factors of type 1 immune response and NK cell activation. Fish Shellfish Immunol. 2020;107(Pt A):104-117. doi:10.1016/j.fsi.2020.09.029(IF:4.581)
[55] Wu Y, Yang Y, Dang H, et al. Molecular identification of Klebsiella pneumoniae and expression of immune genes in infected spotted gar Lepisosteus oculatus. Fish Shellfish Immunol. 2021;119:220-230. doi:10.1016/j.fsi.2021.10.002(IF:4.581)
[56] Wu L, Gao A, Lei Y, Li J, Mai K, Ye J. SHP1 tyrosine phosphatase gets involved in host defense against Streptococcus agalactiae infection and BCR signaling pathway in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020;99:562-571. doi:10.1016/j.fsi.2020.02.026(IF:4.581)
[57] Lv J, Dong T, Zhang Y, et al. Metabolomic profiling of brassinolide and abscisic acid in response to high-temperature stress. Plant Cell Rep. 2022;41(4):935-946. doi:10.1007/s00299-022-02829-2(IF:4.570)
[58] Cao Y, Lu Z, Wang D, et al. Therapeutic evaluation and metabolic reprograming of isosteviol sodium in a rat model of ischemic cardiomyopathy. Eur J Pharmacol. 2021;911:174539. doi:10.1016/j.ejphar.2021.174539(IF:4.432)
[59] Long J, Liu L, Zhou X, Lu X, Qin L. HLA-DQB1-AS1 Promotes Cell Proliferation, Inhibits Apoptosis, and Binds with ZRANB2 Protein in Hepatocellular Carcinoma. J Oncol. 2022;2022:7130634. Published 2022 May 11. doi:10.1155/2022/7130634(IF:4.375)
[60] Chen G, Fan X, Li Y, et al. Promoter aberrant methylation status of ADRA1A is associated with hepatocellular carcinoma. Epigenetics. 2020;15(6-7):684-701. doi:10.1080/15592294.2019.1709267(IF:4.251)
[61] Zhang P, Li H, Zhou C, et al. Single-Cell RNA Transcriptomics Reveals the State of Hepatic Lymphatic Endothelial Cells in Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. J Clin Med. 2022;11(10):2910. Published 2022 May 20. doi:10.3390/jcm11102910(IF:4.242)
[62] Zhang X, Qi W, Shi Y, et al. Role of miR-145-5p/ CD40 in the inflammation and apoptosis of HUVECs induced by PM2.5. Toxicology. 2021;464:152993. doi:10.1016/j.tox.2021.152993(IF:4.221)
[63] Zhou X, Li S, Yang X. The DcPS1 cooperates with OSDLa during pollen development and 2n gamete production in carnation meiosis. BMC Plant Biol. 2022;22(1):259. Published 2022 May 24. doi:10.1186/s12870-022-03648-z(IF:4.215)
[64] Jia H, Zhang Z, Zhang S, et al. Effect of the Methylation Level on the Grape Fruit Development Process. J Agric Food Chem. 2020;68(7):2099-2115. doi:10.1021/acs.jafc.9b07740(IF:4.192)
[65] Shishay G, Liu G, Jiang X, et al. Variation in the Promoter Region of the MC4R Gene Elucidates the Association of Body Measurement Traits in Hu Sheep. Int J Mol Sci. 2019;20(2):240. Published 2019 Jan 9. doi:10.3390/ijms20020240(IF:4.183)
[66] Sun Y, Gao L, Yuan M, Yuan L, Yang J, Zeng T. In vitro and in vivo Study of Antifungal Effect of Pyrvinium Pamoate Alone and in Combination With Azoles Against Exophiala dermatitidis. Front Cell Infect Microbiol. 2020;10:576975. Published 2020 Oct 23. doi:10.3389/fcimb.2020.576975(IF:4.123)
[67] Sun Y, Gao L, Yuan M, Yuan L, Yang J, Zeng T. In vitro and in vivo Study of Antifungal Effect of Pyrvinium Pamoate Alone and in Combination With Azoles Against Exophiala dermatitidis. Front Cell Infect Microbiol. 2020;10:576975. Published 2020 Oct 23. doi:10.3389/fcimb.2020.576975(IF:4.123)
[68] Yang Y, Zhu X, Cui R, et al. Identification of soybean phosphorous efficiency QTLs and genes using chlorophyll fluorescence parameters through GWAS and RNA-seq. Planta. 2021;254(6):110. Published 2021 Oct 30. doi:10.1007/s00425-021-03760-8(IF:4.116)
[69] Sun T, Zhang L, Feng J, et al. Characterization of cellular senescence in doxorubicin-induced aging mice. Exp Gerontol. 2022;163:111800. doi:10.1016/j.exger.2022.111800(IF:4.032)
[70] Wang Z, Yang X, Kai J, et al. HIF-1α-upregulated lncRNA-H19 regulates lipid droplet metabolism through the AMPKα pathway in hepatic stellate cells. Life Sci. 2020;255:117818. doi:10.1016/j.lfs.2020.117818(IF:3.647)
[71] Wang J, Jia Z, Dang H, Zou J. Meteorin-like/Meteorin-β upregulates proinflammatory cytokines via NF-κB pathway in grass carp Ctenopharyngodon idella. Dev Comp Immunol. 2022;127:104289. doi:10.1016/j.dci.2021.104289(IF:3.636)
[72] Wei W, Wang J, Min Q, et al. CCL19 variants mediate chemotactic response via CCR7 in grass carp Ctenopharyngodon idella. Dev Comp Immunol. 2021;122:104127. doi:10.1016/j.dci.2021.104127(IF:3.636)
[73] Yin X, Bai H, Mu L, et al. Expression and functional characterization of the mannose receptor (MR) from Nile tilapia (Oreochromis niloticus) in response to bacterial infection. Dev Comp Immunol. 2022;126:104257. doi:10.1016/j.dci.2021.104257(IF:3.636)
[74] Xu YH, Feng YF, Zou R, Yuan F, Yuan YZ. Silencing of YAP attenuates pericyte-myofibroblast transition and subretinal fibrosis in experimental model of choroidal neovascularization. Cell Biol Int. 2022;46(8):1249-1263. doi:10.1002/cbin.11809(IF:3.612)
[75] Qiao K, Wang F, Liang S, Wang H, Hu Z, Chai T. New Biofortification Tool: Wheat TaCNR5 Enhances Zinc and Manganese Tolerance and Increases Zinc and Manganese Accumulation in Rice Grains. J Agric Food Chem. 2019;67(35):9877-9884. doi:10.1021/acs.jafc.9b04210(IF:3.571)
[76] Yang Y, Wang L, Che Z, et al. Novel target sites for soybean yield enhancement by photosynthesis. J Plant Physiol. 2022;268:153580. doi:10.1016/j.jplph.2021.153580(IF:3.549)
[77] Ji X, Tang Z, Shuai W, et al. Endogenous peptide LYENRL prevents the activation of hypertrophic scar-derived fibroblasts by inhibiting the TGF-β1/Smad pathway. Life Sci. 2019;231:116674. doi:10.1016/j.lfs.2019.116674(IF:3.448)
[78] Hu F, Li C, Zheng X, et al. Lung adenocarcinoma resistance to therapy with EGFR‑tyrosine kinase inhibitors is related to increased expression of cancer stem cell markers SOX2, OCT4 and NANOG. Oncol Rep. 2020;43(2):727-735. doi:10.3892/or.2019.7454(IF:3.417)
[79] Yang M, Yarra R, Zhang R, et al. Transcriptome analysis of oil palm pistil during pollination and fertilization to unravel the role of phytohormone biosynthesis and signaling genes. Funct Integr Genomics. 2022;22(2):261-278. doi:10.1007/s10142-022-00834-y(IF:3.410)
[80] Wu M, Sun X, Wang T, Zhang M, Li P. TRPS1 knockdown inhibits angiogenic vascular mimicry in human triple negative breast cancer cells. Clin Transl Oncol. 2022;24(1):145-153. doi:10.1007/s12094-021-02676-9(IF:3.405)
[81] Zhang H, Yang X, Hu F, et al. Expression Level of Wnt5a Was Related to the Therapeutic Effects of First-Generation EGFR-TKIs. Onco Targets Ther. 2020;13:5387-5394. Published 2020 Jun 11. doi:10.2147/OTT.S250024(IF:3.337)
[82] Xue Y, Jiang X, Gao J, et al. Functional characterisation of interleukin 34 in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2019;92:91-100. doi:10.1016/j.fsi.2019.05.059(IF:3.298)
[83] Chen S, Li M, Jiang W, Zheng H, Qi LW, Jiang S. The role of Neu1 in the protective effect of dipsacoside B on acetaminophen-induced liver injury. Ann Transl Med. 2020;8(13):823. doi:10.21037/atm-19-3850(IF:3.297)
[84] Yang B, Wang Y, Qin Q, et al. Polychlorinated Biphenyl Quinone Promotes Macrophage-Derived Foam Cell Formation. Chem Res Toxicol. 2019;32(12):2422-2432. doi:10.1021/acs.chemrestox.9b00184(IF:3.274)
[85] Liao X, Jin R, Zhang X, et al. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens (Stål). Pest Manag Sci. 2019;75(6):1646-1654. doi:10.1002/ps.5282(IF:3.255)
[86] Zhuo Y, Guo Z, Ba T, et al. African Swine Fever Virus MGF360-12L Inhibits Type I Interferon Production by Blocking the Interaction of Importin α and NF-κB Signaling Pathway. Virol Sin. 2021;36(2):176-186. doi:10.1007/s12250-020-00304-4(IF:3.242)
[87] Fan X, Guo H, Dai B, He L, Zhou D, Lin H. The association between methylation patterns of DNAH17 and clinicopathological factors in hepatocellular carcinoma. Cancer Med. 2019;8(1):337-350. doi:10.1002/cam4.1930(IF:3.202)
[88] Gao J, Jiang X, Wang J, et al. Phylogeny and expression modulation of interleukin 1 receptors in grass carp (Ctenopharyngodon idella). Dev Comp Immunol. 2019;99:103401. doi:10.1016/j.dci.2019.103401(IF:3.119)
[89] Li Q, Wang Y, Pan Y, Wang J, Yu W, Wang X. Unraveling synonymous and deep intronic variants causing aberrant splicing in two genetically undiagnosed epilepsy families. BMC Med Genomics. 2021;14(1):152. Published 2021 Jun 9. doi:10.1186/s12920-021-01008-8(IF:3.063)
[90] Zhang Y, Zhu Z, Ding H, et al. β-catenin stimulates Tcf7l1 degradation through recruitment of casein kinase 2 in mouse embryonic stem cells. Biochem Biophys Res Commun. 2020;524(2):280-287. doi:10.1016/j.bbrc.2020.01.074(IF:2.985)
[91] Qi J, Wu Q, Cheng Q, Chen X, Zhu M, Miao C. High Glucose Induces Endothelial COX2 and iNOS Expression via Inhibition of Monomethyltransferase SETD8 Expression. J Diabetes Res. 2020;2020:2308520. Published 2020 Feb 23. doi:10.1155/2020/2308520(IF:2.965)
[92] Chong Y, Liu G, Girmay S, Jiang X. Novel mutations in the signal transducer and activator of transcription 3 gene are associated with sheep body weight and fatness traits. Mamm Genome. 2021;32(1):38-49. doi:10.1007/s00335-020-09850-4(IF:2.957)
[93] Ma Z, Li F, Chen L, et al. Autophagy promotes hepatic differentiation of hepatic progenitor cells by regulating the Wnt/β-catenin signaling pathway. J Mol Histol. 2019;50(1):75-90. doi:10.1007/s10735-018-9808-x(IF:2.937)
[94] Ali E, Mao K, Liao X, Jin R, Li J. Cross-resistance and biochemical characterization of buprofezin resistance in the white-backed planthopper, Sogatella furcifera (Horvath). Pestic Biochem Physiol. 2019;158:47-53. doi:10.1016/j.pestbp.2019.04.008(IF:2.870)
[95] Dong Y, Liu J, Xue Z, et al. Pao Pereira extract suppresses benign prostatic hyperplasia by inhibiting inflammation-associated NFκB signaling. BMC Complement Med Ther. 2020;20(1):150. Published 2020 May 16. doi:10.1186/s12906-020-02943-2(IF:2.833)
[96] Duan X, Zhao B, Jin X, Cheng X, Huang S, Li J. Antibiotic Treatment Decrease the Fitness of Honeybee (Apis mellifera) Larvae. Insects. 2021;12(4):301. Published 2021 Mar 30. doi:10.3390/insects12040301(IF:2.769)
[97] Wang C, Zhao F, Li Z, et al. Effects of Resveratrol on Growth Performance, Intestinal Development, and Antioxidant Status of Broilers under Heat Stress. Animals (Basel). 2021;11(5):1427. Published 2021 May 17. doi:10.3390/ani11051427(IF:2.752)
[98] Wang N, Zeng GZ, Yin JL, Bian ZX. Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun. 2019;519(3):533-539. doi:10.1016/j.bbrc.2019.09.023(IF:2.705)
[99] Chen L, Zhang M, Wang X, et al. Cardiac steroid ouabain transcriptionally increases human leukocyte antigen DR expression on monocytes. Steroids. 2021;175:108915. doi:10.1016/j.steroids.2021.108915(IF:2.668)
[100] Huang J, Guo M, Jin S, et al. Antibacterial photodynamic therapy mediated by 5-aminolevulinic acid on methicillin-resistant Staphylococcus aureus. Photodiagnosis Photodyn Ther. 2019;28:330-337. doi:10.1016/j.pdpdt.2019.09.008(IF:2.589)
[101] Gao M, Guo L, Wang H, et al. Orphan nuclear receptor RORγ confers doxorubicin resistance in prostate cancer. Cell Biol Int. 2020;44(10):2170-2176. doi:10.1002/cbin.11411(IF:2.571)
[102] Chen DD, Ahmad M, Liu YH, et al. Transcriptomic responses of haloalkalitolerant bacterium Egicoccus halophilus EGI 80432T to highly alkaline stress. Extremophiles. 2021;25(5-6):459-470. doi:10.1007/s00792-021-01239-8(IF:2.395)
[103] Jiao Z, Zhu X, Li H, et al. Cytological and molecular characterizations of a novel 2A nullisomic line derived from a widely-grown wheat cultivar Zhoumai 18 conferring male sterility. PeerJ. 2020;8:e10275. Published 2020 Oct 30. doi:10.7717/peerj.10275(IF:2.379)
[104] Guo Z, Qiu C, Mecca C, et al. Elevated lymphotoxin-α (TNFβ) is associated with intervertebral disc degeneration. BMC Musculoskelet Disord. 2021;22(1):77. Published 2021 Jan 13. doi:10.1186/s12891-020-03934-7(IF:2.355)
[105] You L, Chen H, Xu L, Li X. Overexpression of miR-29a-3p Suppresses Proliferation, Migration, and Invasion of Vascular Smooth Muscle Cells in Atherosclerosis via Targeting TNFRSF1A. Biomed Res Int. 2020;2020:9627974. Published 2020 Sep 4. doi:10.1155/2020/9627974(IF:2.276)
[106] Liang X, Yan F, Gao Y, et al. Sugar transporter genes in grass carp (Ctenopharyngodon idellus): molecular cloning, characterization, and expression in response to different stocking densities. Fish Physiol Biochem. 2020;46(3):1039-1052. doi:10.1007/s10695-020-00770-3(IF:2.249)
[107] Li Z, Cai T, Qin Y, et al. Transcriptional Response of ATP-Binding Cassette (ABC) Transporters to Insecticide in the Brown Planthopper, Nilaparvata lugens (Stål). Insects. 2020;11(5):280. Published 2020 May 2. doi:10.3390/insects11050280(IF:2.220)
[108] Wang M, Wan H, Wang S, et al. RSK3 mediates necroptosis by regulating phosphorylation of RIP3 in rat retinal ganglion cells. J Anat. 2020;237(1):29-47. doi:10.1111/joa.13185(IF:2.013)